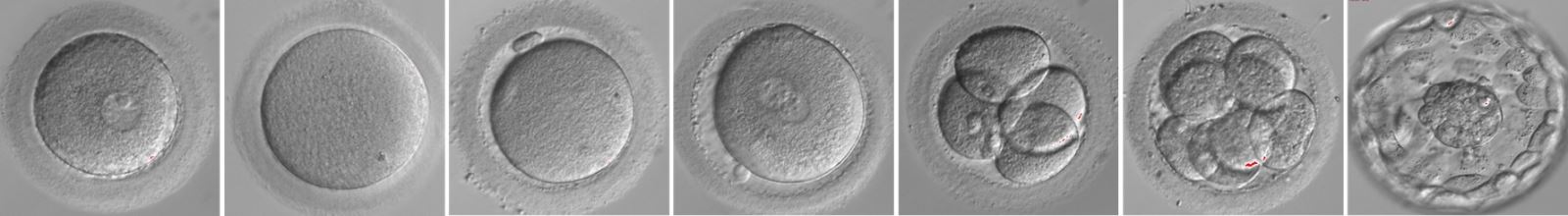
1. Genetics of human oocyte maturation arrest
Oocyte maturation occurs through a series of molecular and morphological changes. Oocytes are initially arrested at the diplotene stage of prophase I, at which point they are referred to as germinal vesicle (GV) oocytes. Upon a surge in luteinizing hormone, GV oocytes exit prophase I and resume meiosis, and this is followed by chromatin condensation and nuclear envelope breakdown. Coupled with spindle formation and chromosome alignment, oocytes enter into meiosis I (referred to as MI oocytes). Meiosis I is completed by extruding the first polar body. Oocytes then enter into meiosis II (referred to as MII oocytes) but arrest at metaphase of meiosis II until fertilization. Only mature oocytes can be fertilized, thus ensuring that embryos can be developed. In clinical in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) attempts, oocyte maturation arrest can occur at different stages, including GV and MI oocytes, leading to female infertility. The genetic basis for oocyte maturation arrest was previously unknown, but altogether we have discovered four new Mendelian diseases, including oocyte GV arrest, oocyte MI arrest, zygote arrest, and oocyte death. We identified several novel mutant genes related to these phenotypes, including TUBB8 (N Engl J Med, 2016);PATL2 (Am J Hum Genet, 2017);WEE2 (Am J Hum Genet, 2018);PANX1 (Sci Transl Med, 2019);TRIP13 (Am J Hum Genet, 2020);LHX8 (Genet Med, 2022);PABPC1L (EMBO Mol Med, 2023);COX15 (Proc Natl Acad Sci U S A, 2024).We have clarified pathogenic mechanisms and explored intervention strategies. Although these genes can account for a certain percentage of patients, the genetic factors for most of patients remain unknown. We are currently trying to identify other disease-causing genes and to investigate the corresponding mechanisms.
2. Genetics of early embryonic arrest
In an IVF or ICSI cycle, mature oocytes are retrieved and normal fertilized oocytes continue to be cultured. Embryo quality is assessed 3 days after fertilization on the basis of cell number and morphology because normal embryonic development is the key to establishing a successful pregnancy. IVF and ICSI cycles fail, if all of an individual's embryos are arrested at early stages of development. Some infertile individuals have this phenotype after multiple failed IVF and ICSI attempts. However, the genetic determinants that cause early human embryonic arrest have remained largely unknown. We have identified a few disease-causing genes, including PADI6 (Am J Hum Genet, 2017);BTG4 (Am J Hum Genet, 2020), CDC20 (Protein Cell, 2020);KPNA7 (J Clin Invest, 2023);TUBA4A (Genome Biol, 2023) and have revealed the corresponding mechanisms. As a result of the discovery of the series of disease-causing genes, our team was invited to publish a review in Science (Science, 2023). However, these genes can only explain the genetic cause of a small number of patients, and we are currently trying to identify other genetic factors related to this disease phenotype.
3. Molecular mechanism of oocyte maturation and early embryonic development.
By using 3D fluorescence high resolution live cell imaging and functional genomics in human oocytes, we discovered the unique human oocyte microtubule organizing center (huoMTOC) that is responsible for initiating spindle assembly in oocytes (Science, 2022). We have further revealed the unique mechanism of spindle bipolarization (Science, 2024) and elucidated the mechanism of occurrence of high percentage of aneuploidy in human oocytes (Cell Discov, 2023).We are currently elucidating the mechanisms behind spindle assembly and are studying the corresponding genes' roles in spindle assembly, oocyte maturation, and early embryonic development. We hope to identify additional physiological mechanisms behind human/mouse spindle assembly, oocyte maturation, and embryonic development.
©复旦大学生殖与遗传团队 All rights reserved.

 Chinese
Chinese